Are there patterns in how a variable surface protein family binds to its human receptors?
Our previous study of how PfEMP1 proteins bind to their human receptor EPCR (Lau et al, 2015), showed that they use a site of variable sequence, but conserved shape and chemistry to bind to a functionally important site on their human receptor. This is a winning strategy for the parasite as it allows variation to avoid immune detection, while targeting a site on the human host which cannot be varied without losing an important function. Is this a common strategy in malaria?
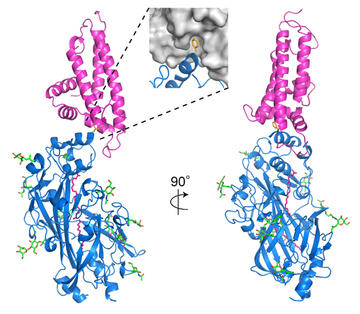
To investigate this, Fu-Lien Hsieh decided to study how PfEMP1 proteins bind to a second ligand, CD36. Fu determined a crystal structure of a complex of a PfEMP1 domain bound to CD36 and, with Thomas Lavstsen and colleagues, explored sequence variation in this site. Once again, significant variation in the ligand binding residues of the PfEMP1 was seen. But once again, a site of conserved chemistry and shape marked the binding site, this time with a hydrophobic pocket surrounded by a hydrophilic surface of conserved shape.
But do the PfEMP1 bind to a functionally important site on CD36? One of the functions of CD36 is to bind to lipoprotein particles as it scavenges fatty acids for the cell. Fu showed that a mutation of CD36 which prevents PfEMP1 binding also blocks lipoprotein binding. Once again, the PfEMP1 targets a functionally important site of this human receptor.
PfEMP1 that bind to EPCR and CD36 experience the same conflicting selection pressures, to diversify to avoid immune detection while retaining the ability to bind to a human receptor. Both have solved this problem using a sequence diverse surface of conserved shape and chemistry to bind a functionally important site on their human receptor.
Hsieh, F., Turner, L., Bolla, J.L., Robinson, C.V., Lavstsen, T. and Higgins, M.K. (2016) The structural basis for CD36 binding by the malaria parasite. Nature Comm 7 12837