An eon in the life of a trypanosome receptor
A receptor protein at the front line of the battle between host and pathogen
Parasites are adaptable. If they can survive the onslaught of the immune defences of their chosen host organism, then they can benefit from protection and access to rich sources of nutrition. But if they are detected, then they will be destroyed.
At the front line in this battle are receptor proteins. These molecules decorate the parasite surface allowing the parasite to acquire essential nutrients. But these receptors must avoid detection or they will signal the presence of the parasite to the host immune defences.
The trypanosomes, which cause diseases such as nagana in cattle and sleeping sickness in humans, live free in the blood of infected mammals. How do their receptor proteins function without compromising the safety of the parasite, and how adaptable are they? Our studies of the haptoglobin-haemoglobin receptor, done in close collaboration with Mark Carrington, reveal a remarkable story of molecular adaptation as this receptor has adopted very different roles in different trypanosome species.
A detailed view of the Trypanosoma brucei haptoglobin-haemoglobin receptor
The first trypanosome haptoglobin-haemoglobin receptor was identified in Trypanosoma brucei. It is expressed by trypanosomes living within human blood and allows the parasite to acquire the nutrient haem. Much of the haem found in mammalian blood is bound to haemoglobin. But this oxygen carrier is held safely within red blood cells, inaccessible to the trypanosome. When these blood cells rupture or become damaged, the released haemoglobin is inactivated by binding to haptoglobin, forming a complex found free in serum. The receptor is able to grab hold of haptoglobin-haemoglobin, but not haemoglobin alone, allowing the parasite to harvest haem.
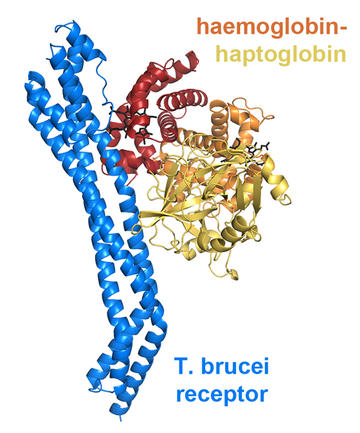
To see how this receptor works, we determined the molecular structure of its complex with a monomeric form of haptoglobin-haemoglobin. It is a slender, elongated receptor with its ligand binding site at one end. The receptor contacts both haptoglobin and haemoglobin, as well as directly binding to haem molecules. This ensures that it specifically selects haem-loaded haptoglobin-haemoglobin complexes for uptake.
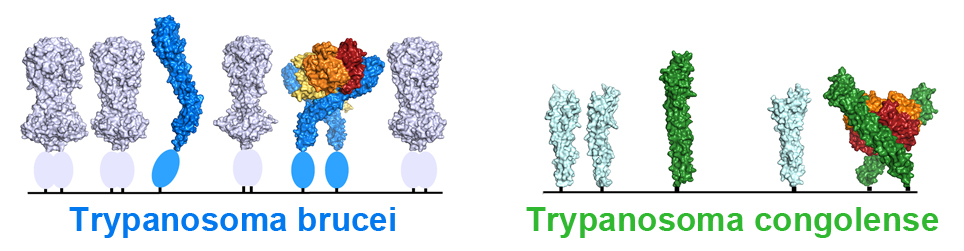
A striking feature of the receptor is a kink part way along its length. This is likely to be an adaptation to allow it to function on the densely packed trypanosome surface. Trypanosomes have evolved a remarkable coat, packed with many copies of a VSG molecule. This can be switched to a different VSG molecule when detected by the immune system. The receptor must operate within this surface layer, and yet the binding site for haptoglobin-haemoglobin lies along one side of the receptor, risking crowding from the VSGs. But the kink helps solve this problem, holding apart the VSG molecules surrounding the receptor and keeping the binding site open.
The kink also has a second role. Haptoglobin-haemoglobin in the blood assembles into a dimer and modelling the dimeric complex shows that the kink allows two membrane-attached receptors to simultaneously bind to a single haptoglobin-haemoglobin dimer. Indeed, trypanosomes more efficiently take up dimeric haptoglobin-haemoglobin than the monomeric form.
So, Trypanosoma brucei acquires haem from mammalian serum using a receptor which is highly adapted to operate in the blood stream form of the trypanosome. Is the same true in other trypanosome species?
A haemoglobin receptor in Trypanosoma congolense
Our studies of the haptoglobin-haemoglobin receptor of Trypanosoma congolense revealed a number of surprises. Firstly, while the receptor still binds to haptoglobin-haemoglobin, it binds much more tightly to haemoglobin. Why is this? Free haemoglobin is not found in the blood.
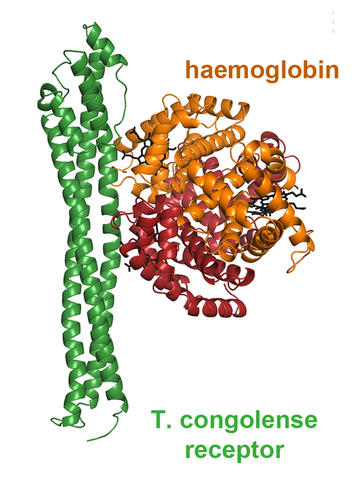
Second, the receptor lacks the kink which we believe is required for it to function within the VSG layer on the blood stream form trypanosome surface. How does it keep its binding site accessible in the absence of this kink?
The answer came from a third surprise. In Trypanosoma congolense, the receptor is no longer expressed in the blood stream of infected mammals. Instead it is expressed when the parasite lives within the mouthparts of the tsetse fly. Therefore, rather than acquiring haem in the form of haptoglobin-haemoglobin from serum, it must acquire haem from the blood meals taken by the fly. Here, the red blood cells will burst, releasing free haemoglobin galore. In this environment, it is much better to have a haemoglobin receptor.
This also explains why the receptor lacks the kink. The surface of the trypanosome in the tsetse fly mouthparts lacks the dense VSG coat. The kink is no longer required to hold apart VSG molecules and to keep open the ligand binding site. And, with the change in ligand, the kink is no longer needed to allow two receptors to bind tightly to a single haptoglobin-haemoglobin molecule.
An evolutionary journey: what came first?
So, what came first? Was it a blood stream haptoglobin-haemoglobin receptor or an insect-expressed haemoglobin receptor? We found that the receptor from a third trypanosome species, Trypanosoma vivax, binds preferentially to haemoglobin. Together with analysis of the evolutionary tree of the trypanosomes, this suggests that the haemoglobin receptor came first. In Trypanosoma brucei, the receptor then changed where it is expressed and what ligand it binds, as well as adapting its structure to its new context. In one small story of one receptor, this highlights the adaptability of the essential machinery of the parasite surface to allow the parasite to survive and flourish within its different hosts.
To find out more:
Higgins, M.K.*, Lane-Serff, H., MacGregor, P. and Carrington, M.* (2017) A receptor’s tale: an eon in the life of a trypanosome receptor. PLoS Pathogen 13 e1006055
With the original research found in:
Lane-Serff, H., MacGregor, P., Peacock, L., Macleod, O.J., Kay, C., Gibson, W., Higgins, M.K.* and Carrington, M. * (2016) Evolutionary diversification of the trypanosome haptoglobin-haemoglobin receptor from an ancestral haemoglobin receptor. eLife 5 e13044
Lane-Serff, H., McGregor, P., Lowe, E.D., Carrington, M.* and Higgins, M.K.* (2014) Structural basis for ligand and innate immunity factor uptake by the trypanosome haptoglobin-haemoglobin receptor. eLife 3 e05553
Higgins M.K.*, Tkachenko O., Brown A., Reed J., Raper J. and Carrington M* (2013) Structure of the trypanosome haptoglobin-hemoglobin receptor and implications for nutrient uptake and innate immunity. Proc. Natl. Acad. Sci USA 110 1905-1910.


