Our blood is packed full of haemoglobin, which plays a critical role, carrying oxygen from our lungs to our muscles and tissues. Haemoglobin is carried securely in our red blood cells, but if released, it is dangerous and causes oxidative damage. But what happens when our blood cells burst? To protect us, our blood contains a system for detoxifying free haemoglobin.
The first step in haemoglobin detoxification involves an abundant blood protein called haptoglobin. This binds tightly to free haemoglobin, forming the haptoglobin-haemoglobin complex. These HpHb complexes are cleared from the blood by macrophages, which contain a scavenger receptor, CD163 which selectively binds to HpHb. HpHb from different individuals has different shapes and structures, but CD163 must recognise all of these forms. It is also important that CD163 does not take up haptoglobin without haemoglobin as this would deplete this important molecule from our blood without detoxifying haemoglobin. How does it do it?
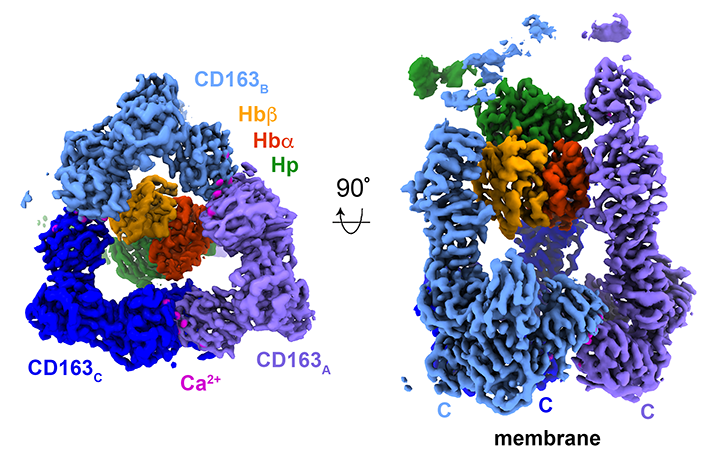
Richard Zhou was able to show how CD163 binds to HpHb and he unveiled some big surprises. He used cryogenic electron microscopy to determine the structure of CD163 bound to HpHb. Unexpected three CD163 molecules assemble, through interactions mediated by calcium, to form a triangular base. Protruding from this base are three arms, which come together to hold a single HpHb. CD163 also binds to other ligands including haemoglobin. The use of the three arms to create a binding site allows CD163 to be promiscuous, moulding around different ligands to form different binding sites.
Richard designed a version of CD163 which is unable to form trimers and assessed the ability of this monomeric receptor to take different ligands into cells. In humans, HpHb comes in varying forms depending on our genetic type. These have the same ‘head’, containing haemoglobin and part of haptoglobin, but differ in the number of these heads which are linked together. The trimeric form of CD163 allows all forms to be taken up equally, while also allowing less efficient uptake of free haemoglobin. In contrast, the monomeric receptor takes up forms of HpHb containing more heads with greater efficiency that those with fewer heads and barely takes up any free haemoglobin. Therefore, assembly of trimeric CD163 gives the receptor the ability to efficiently take up ligands with different architectures.
Richard also showed that the arms of CD163 can bind to each other when ligand is absent and that the interactions which the arms make with each other use the same surfaces which they use to interact with HpHb. This will allow CD163 to inhibit ligand binding through these arm-arm interactions. Ligands will only bind if they are able to compete for arm-arm binding, reducing the degree to which CD163 is promiscuous.
Finally, Richard could reveal the role of calcium. Calcium ions are present both in the binding site, between CD163 and HpHb, as well as in the interfaces which lead to trimerization. The blood and serum contain enough calcium to allow trimeric receptor complexes to form and to bind ligand. In contrast, in the endosomes, calcium levels are low and HpHb will monomerise and release HpHb to be degraded and detoxified.
This study therefore shows how a scavenger receptor can selectively allow uptake of a range of different ligands, protecting our bodies from oxidative damage from free haemoglobin.
Zhou, R.X. and Higgins, M.K. (2024) Scavenger receptor CD163 multimerises to allow uptake of diverse ligands. Nature Communications 16 6623