Two strategies for survival used by trypanosome receptors
Living under constant barrage
Trypanosomes live free in the blood of mammals. This provides huge opportunity as they are constantly surrounded by rich sources of nutrition. But it is also a huge challenge. They must survive under constant attack from molecules of the human immune system. Antibody-mediated recognition could be the end of them.
To survive within enemy territory, trypanosomes have evolved a remarkable surface coat. A layer of VSG molecules forms a dense and protective covering. Each trypanosome has a repertoire of VSGs and they can change their coat by switching which of these they use. This helps them to stay under cover.
To allow trypanosomes to exploit the nutrients in their environment, receptors operate within this VSG layer. These must recognise nutrient molecules from the serum, without revealing the presence of the trypanosome to the host. Our work with Mark Carrington’s group has revealed the very different strategies used by two different trypanosomes receptors.

The haptoglobin-haemoglobin receptor: a slender slip of a thing
The haptoglobin-haemoglobin receptor is used by trypanosomes to acquire the nutrient haem, found in the serum within its carrier haptoglobin-haemoglobin. Our structure of the receptor bound to its ligand gives clues about how it operates.
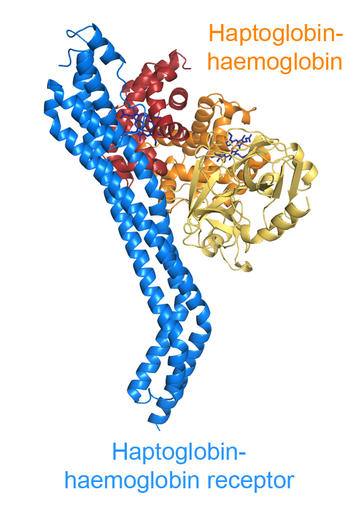
A quick glance reveals that the receptor is slender. This will allow it to slip in between closely packed VSG molecules with only its tip exposed and with the majority of the receptor hidden away from detection.
However, the nutrient binding site does not lie on this small exposed tip, but is instead found on the side of the receptor. This would leave it closed in by the VSG layer if it were not for a cunning adaptation. The receptor is kinked one third of the way along its length, holding apart the neighbouring VSG molecules. It is the receptor binding surface which will have its accessibility increased because of the location of this kink.
The kink has a second important role. The haptoglobin-haemoglobin molecule is a dimer, and the kink positions the binding site so that two membrane-associated receptors can simultaneously bind to the two binding sites on the dimeric nutrient. This helps the trypanosome to hold on more tightly to the nutrient molecule so it can be more efficiently taken up.
This slender receptor therefore snuggles into the VSG layer, exposing little of its surface. But a kink allows enough exposure of its ligand binding site to allow it to help the trypanosome to this useful nutrient.
The transferrin receptor(s): a family affair
In contrast, the receptor for transferrin, a rich source of iron, is not slender. It has broad shoulders, which will hold apart VSG molecules to present a large binding platform to bind to its nutrient. Indeed, not satisfied with its own breadth, it displays glycan molecules around its circumference, holding the VSGs even further apart.

This has consequences. Presenting a large platform for ligand binding to the blood will also expose the receptor, making it more likely to be detected by the mammalian defences. How does it avoid being seen?
It appears as though the receptor uses a trick borrowed from the VSGs. The trypanosome doesn’t just have one transferrin receptor, but has a set. This will allow it to perform the trick of antigenic variation. As the infected mammal learns to recognise one transferrin receptor, the trypanosome can change its coat, and use a different transferrin receptor instead.
Is this what happens? Transferrin receptor switching to help evasion of immune detection has not yet been directly observed. However, looking at how the different transferrin receptors available to the parasite differ from one another supports this idea. The regions of the receptor most exposed to antibody binding are the regions which differ the most.
Two strategies
These two receptors have evolved two very different strategies. The haptoglobin-haemoglobin receptor stays thin, slipping between the VSGs and making itself as invisible as possible to the immune system. On the other hand, the transferrin receptor exposes a large platform to which transferrin binds. To aid survival, it is part of a family and, when recognised, it can disappear, only to be replaced by a cousin who the mammalian immune system does not yet recognise. These two receptors use very different strategies to allow them to work unseen. How many other strategies are there for other trypanosome receptors?
To find out more:
Trevor, C., Gonzalez-Munoz, A.L., MacLeod, O.J.S., Woodcock, P.G., Rust, S., Vaughan, T.J., Garman, E.F., Minter, R., Carrington, M.* and Higgins, M.K.* (2019) Structure of the trypanosome transferrin receptor reveals mechanisms of ligand recognition and immune evasion. Nature Microbiology 4 2074-2081
Lane-Serff, H., McGregor, P., Lowe, E.D., Carrington, M.* and Higgins, M.K.* (2014) Structural basis for ligand and innate immunity factor uptake by the trypanosome haptoglobin-haemoglobin receptor. eLife 3 e05553


