Understanding the molecular machinery which enables the malaria parasite to invade blood cells
An active parasite that drives its way into blood cells
The malaria parasite makes a home inside our cells. This allows it to hide away from detection by our immune system and find a safe place to replicate. But it also provides a challenge. How does it get inside a human cell?
Malaria parasites replicate within our red blood cells. These are unusually simple cells, which lack many of the pieces of molecular and cellular machinery which most of our other cells have. To get inside a red blood cell, the malaria parasite must be extremely active, driving its way in and they have evolved a large array of molecular machinery to achieve this. How do they do it?
We have mostly focused on understanding the molecular machinery used by the most-deadly human-infective malaria parasite, Plasmodium falciparum. As well as unveiling a fascinating piece of parasite biology, we aim that these studies will help us to develop improved vaccine immunogens as we help to tackle the scourge of malaria.
A crucial red blood cell binding protein
The malaria parasite can use different members of a broad family of surface proteins to interact with human red blood cells. A breakthrough came when Gavin Wright’s team identified that one of these, between the parasite protein RH5 and the red blood cell receptor basigin, was essential for invasion by all tested parasite strains. We wanted to understand how this interaction works.
Using protein crystallography, we revealed that RH5 adopts a kite-like architecture. The top half of RH5 attaches to basigin, forming an
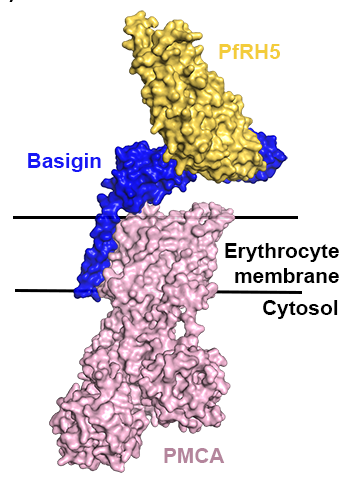
interaction required for invasion. Later, we also showed that basigin is not alone on the red blood cell surface, but forms complexes with two membrane protein receptors, PMCA and MCT1. We showed that RH5 can also bind to these membrane protein complexes. These studies opened the way for many of our studies of how RH5-targeting antibodies function and for design of RH5-based vaccine immunogens.
To form a pore or not?
While we now know how RH5 recognises basigin, we still do not understand why this interaction is required for red blood cell invasion. One theory was that RH5 comes apart and inserts into the red blood cell membrane to form a pore and that this pore is required for invasion.
We therefore conducted a set of experiments to test whether this is the case. Most conclusively, we used our understanding of the structure of RH5 to design some locks which hold it together and prevent the shape changes proposed to lead to pore formation. Parasites were generated in which the standard form of RH5 was replaced with the locked form and this change had no effect on their ability to invade red blood cells. This suggests that RH5 does not need to come apart to form a pore.
If RH5 does not form a pore, then what does it do?
A bridge linking parasite and blood cells
RH5 is not alone on the parasite surface but forms part of a five-component molecular assembly with CyRPA, RIPR, CSS and PTRAMP. Together this is known as the PCRCR complex. Each of these proteins is also essential for the parasite to get inside red
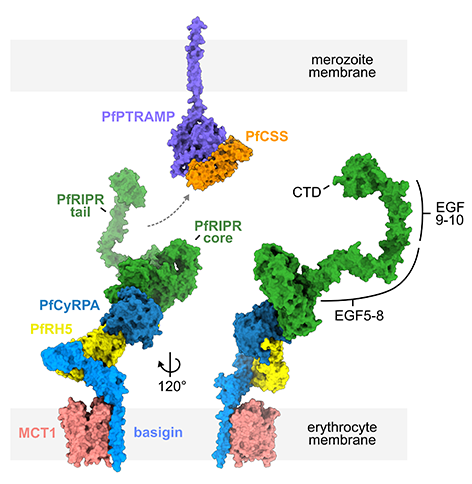
blood cells and we would like to understand what they do.
We started by determining the structure of the RH5:CyRPA:RIPR complex, showing that CyRPA links together RH5 and RIPR. We find that RIPR is formed from an ordered core, which binds to CyRPA, as well as a flexible tail, which interacts with the CSS:PTRAMP complex on the parasite surface. Therefore the PCRCR complex forms a bridge which links basigin on the red blood cell surface through to the parasite surface. Our current hypothesis is that this bridge is essential for the parasite to drive its way into red blood cells. Future studies will reveal how!
To find out more:
Wright K.E., Hjerrild K.A., Bartlett J., Douglas A.D., Jin J., Brown R.E., Illingworth J.J., Ashfield R., Clemmensen S.B., de Jongh W.A., Draper S.J. and Higgins M.K. (2014) Structure of malaria invasion protein RH5 with erythrocyte basigin and blocking antibodies. Nature 515 427-30
Jamwal, A., Constantin, C., Henrich, S., Bildl, W., Fakler, B., Draper, S.J., Schulte, U., Higgins, M.K. (2023) Erythrocyte invasion-neutralising antibodies prevent Plasmodium falciparum RH5 from binding to basigin-containing membrane protein complexes. eLife 12 e83681
Farrell, B., Alam, N., Hart, M.N., Jamwal, A., Ragotte, R.J., Walters-Morgan, H., Draper, S.J., Knuepfer, E. and Higgins, M.K. (2023) Structure of the PfRCR complex which bridges the malaria parasite and erythrocyte during invasion. Nature doi: 10.1038/s41586-023-06856-1


