What do antibodies teach us about how to stop blood stage malaria?
What do antibodies teach us about how to stop blood stage malaria?
Characterising the antibodies generated by human vaccination
The parasites that cause malaria survive and replicate within the red blood cells of infected people. If we can stop them from getting inside red blood cells, we can prevent the symptoms of malaria and can stop transmission of the disease. Vaccines which prevent blood stage malaria by stopping blood cell invasion are therefore a major goal of the international malaria research effort.
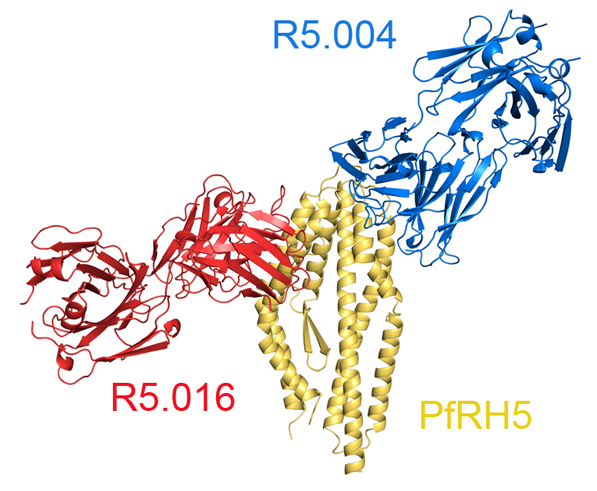
The two major parasites to cause human malaria are Plasmodium falciparum and Plasmodium vivax. In both cases, we know of important pieces of molecular machinery which the parasites use to invade blood cells. For Plasmodium falciparum, this is the RH5 protein, which makes an essential interaction with the human receptor basigin on the blood cell surface. For Plasmodium vivax it is the DBP protein which must bind human DARC receptor. If we can prevent these interactions, we can stop malaria.
Human clinical trials of the safety and efficacy of RH5 and DBP-based vaccines by Simon Draper’s research team provided the opportunity to investigate how the human immune system responds when it sees these two immunogens. Panels of monoclonal antibodies were isolated from immunised human volunteers and were characterised for the ability to prevent parasites from getting into cells. We then used structural methods to understand where these antibodies bind and how they work. What did we discover?
Neutralising antibodies do not all directly block receptor binding sites
First, we find that many of the most effective antibodies directly prevent parasite proteins from binding to their human receptors. As expected, antibodies which bind to RH5 and cover up its basigin binding site, such as antibody R5.004, stop the parasite from getting inside blood cells. Directly inhibitory antibodies often neutralise.
However, many neutralising antibodies are not directly inhibitory of receptor binding. The RH5 binding antibody, R5.016 is one of the
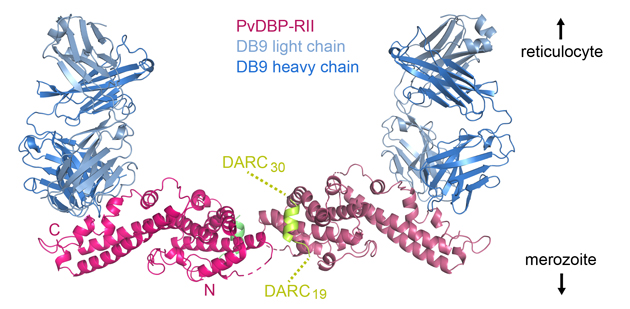
most effective at preventing blood cell invasion but it does not directly block basigin binding. Similarly, the DB9 antibody is very effective at preventing blood cell invasion by Plasmodium vivax, but it does not stop DBP from binding to DARC.
Both RH5 and DBP are linked to the surfaces of invading malaria parasites and both basigin and DARC are attached to blood cell surfaces. It is likely that bulky antibodies attached to these sites on RH5 and DBP will get in the way as the parasite comes close to red blood cells, stopping RH5 from getting close enough to basigin and DBP from getting close enough to DARC to fulfil their functions. Therefore, antibodies which do not directly stop receptor binding, may still be highly effective by indirectly blocking a critical process.
Indeed, we later showed that antibodies like R5.016, do indeed prevent RH5 from binding to basigin in a cellular context. Basigin is not found alone on an infected red blood cell but assembles into larger membrane protein complexes. While R5.016 does not directly stop RH5 from binding to basigin in isolation it does stop RH5 from binding to basigin which is assembled into its natural protein complexes. R5.016 does work by blocking erythrocyte binding by RH5.
Non-neutralising antibodies can be helpful
Second, we find that non-neutralising antibodies can be unexpectedly beneficial. For example, antibody R5.011 binds to RH5 far from the basigin binding site and does not prevent red cell invasion in a growth inhibition assay. However, the presence of R5.011 increases the potency of neutralising antibodies such as R5.004 or R5.016. Even more surprisingly, R5.011 also increases the potency of neutralising antibodies which bind to pieces of the blood cell invasion machinery other than RH5. How?
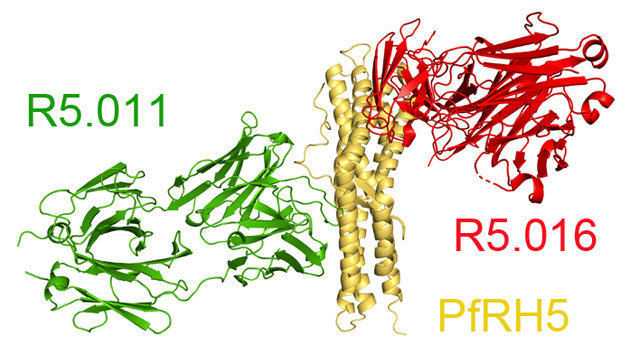
The clue came from studying how quickly parasites invade blood cells. The invasion process is very fast. Video microscopy by Paul Gilson’s group shows that it takes around 20 seconds for the parasite to get into a blood cell. This is a very short window during which an inhibitory antibody can bind to prevent invasion. Antibody R5.011 slows the invasion process by around 4-fold, giving longer for the inhibitory antibodies to act.
Non-neutralising antibodies are often written off in vaccine design strategies. But they can be useful, sometimes in unexpected ways!
Neutralising antibodies can make each other better
A study of monoclonal antibodies targeting CyRPA revealed another way in which antibodies can help each other out. This study identified three different neutralising antibodies, each of which, when provided alone, weakly blocked invasion of erythrocytes. However, when mixed together the combination of these antibodies was substantially better than that expected from adding their effects together.
So how did this synergy work? We used structural studies to see CyRPA bound to all three of these antibodies. This revealed that, as
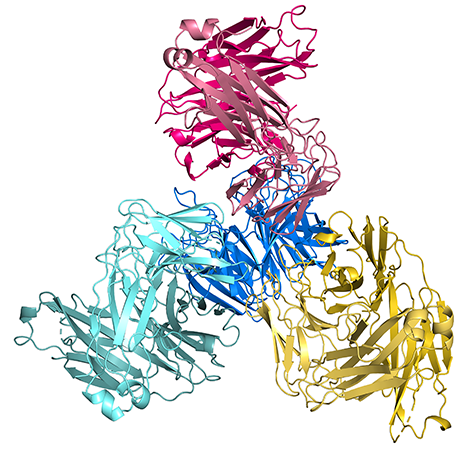
well as binding to CyRPA, these antibodies also bind to each other. This increases their ability to form a stable complex with CyRPA which survives over time. This is the first time that different antibodies have been shown to help each other to bind to a target antigen and to improve efficacy. But we expect that this will prove to be a common mechanism, the exploitation of which will aid vaccine immunogen design.
Non-neutralising antibodies can get in the way
But non-neutralising antibodies can also cause problems. A panel of nine antibodies targeting Plasmodium vivax DBP were tested in combinations. The DB9 antibody was most effective preventing blood cell invasion, but mixing this with five of the other antibodies from the panel reduced its effectiveness. While we do not yet understand the mechanism for this, it demonstrates that production of non-neutralising antibodies is not neutral. They can get in the way.
A blue-print for future vaccine development
The antibody response to vaccination is complex. If we are lucky, it generates highly effective antibodies which neutralise the parasite. But it also generates non-neutralising antibodies. Far from being irrelevant, these may aid or inhibit the action of neutralising antibodies, making them more potent, or alternatively interfering with their function. An ideal vaccine will induce a tailored immune response, which generates only the most effective antibodies, while avoiding anything which interferes with their function. Our goal is now to use our detailed structural knowledge of the epitopes of the most effective antibodies to design a new generation of malaria vaccine components to tackle this ancient scourge with a potent and protective antibody response.
To find out more:
Alanine, D.G.W, Quinkert, D., Kumarasingha, R., Mehmood, S., Donnellan, F.R., Minkah, N.K., Dadonaite, B., Diouf, A., Galaway, F., Silk, S.E., Jamwal, A., Marshall, J.M., Miura, K., Foquet, L., Elias, S.C., Labbé, G.M., Douglas, A.D., Jin, J., Payne, R.O., Illingworth, J., Pattinson, D.J., Pulido-Gomez, D., Williams, B.G., de Jongh, W.A., Wright, G.J., Kappe, S.H.I., Robinson, C.V., Long, C.A., Crabb, B.S., Gilson, P.R., Higgins, M.K.* and Draper, S.J.*. (2019) Human antibodies that slow erythrocyte invasion potentiate malaria-neutralizing antibodies. Cell 178 216-228
Rawlinson, T.A., Barber, N.M., Mohring, F., Cho, J.S., Kosaisavee, V., Gérard, S.F., Alanine, D.G.W., Labbé, G.M., Elias. S.C., Silk, S.E., Quinkert, D., Jin, J., Marshall, J.M., Payne, R.O., Minassian, A.M., Russell, B., Rénia, K., Nosten. F.H., Moon, R.W., Higgins, M.K.* and Draper, S.J.* (2019) Structural basis for inhibition of Plasmodium vivax invasion by a broadly neutralising vaccine-induced human antibody. Nature Microbiology 4 1497-1507
Ragotte, R.J., Pulido, D., Lias, A.M., Quinkert, D., Alanine, D.G.W., Jamwal, A., Davies, H., Nacer, A., Lowe, E.D., Grime, G.W., Illingworth, J.J., Donat, R.F, Garman, E.F., Bowyer, P.W., Higgins, M.K.* and Draper, S.J.* (2022) Heterotypic interactions drive antibody synergy against a malaria vaccine candidate. Nature Communications 13 933
Jamwal, A., Constantin, C., Henrich, S., Bildl, W., Fakler, B., Draper, S.J., Schulte, U., Higgins, M.K. (2022) Erythrocyte invasion-neutralising antibodies prevent Plasmodium falciparum RH5 from binding to basigin-containing membrane protein complexes. BioRXIVdoi/10.1101/2022.09.23.509221v1


